Recently, the research team of Professor Zeng Jie of Hefei National Research Center for Microscale Physical Sciences and School of Chemistry and Materials Science, University of Science and Technology of China, cooperated with Shanghai light source researcher Si Rui, by constructing a Pt monoatomic catalyst supported on a metal-organic framework MIL-101 , Revealing its metal-ligand interaction in the CO2 hydrogenation reaction, this interaction improves the selectivity of CO2 hydrogenation to methanol by regulating the reaction path. The result was titled Optimizing Reaction Paths for Methanol Synthesis from CO2 Hydrogenation via Metal-ligand Cooperativity and was published in Nature-Communication (Nature Commun. 2019, 10, 1885). The co-first authors of the thesis are doctoral students Chen Yizhen and Li Hong Liang and Zhao Wanghui.
In today's society, energy and environmental crises such as depletion of fossil energy and global warming are major problems facing humanity. The CO2 hydrogenation reaction is an important reaction in low-carbon chemistry. On the one hand, it can directly reduce CO2 emissions and alleviate the greenhouse effect; on the other hand, it can synthesize fuels and chemicals to achieve an artificial carbon cycle and ease the shortage of fossil energy. In the actual catalytic process, various reaction paths may coexist, which greatly limits the selectivity of the target product. Therefore, optimizing the reaction path of CO2 hydrogenation is an important strategy for regulating the selectivity and reaction activity of target products.
In response to this problem, the researchers constructed a Pt monoatomic catalyst supported on the metal-organic framework MIL-101, and found that Pt monoatoms form a Pt-OH active center in the CO2 hydrogenation reaction, and the H in the active center Atoms can be added directly to the C-terminus of CO2 as a hydrogen source to form HCOO * intermediates. HCOO * intermediates are not easy to form CO, but easily hydrogenated to form methanol. In contrast, Pt particles will form a Pt-H active center in the CO2 hydrogenation reaction, and the H atoms in the active center will be added to the O end of CO2 to form a COOH * intermediate, and the COOH * intermediate is easy Dehydroxylation forms CO. Therefore, the Pt monoatomic catalyst is at 32 bar and 150. Under the condition of C, it has a methanol selectivity as high as 90.3%, which is much higher than that of Pt particles under the same conditions (13.3%).
This work elaborates the regulation mechanism of metal-ligand interaction in CO2 hydrogenation reaction, and opens a new door for people to better understand the mechanism of single atom catalysis. At the same time, it also opened up new horizons for the strategy of optimizing the CO2 hydrogenation path to increase the activity and selectivity of methanol production.
This research was supported by the National Academy of Sciences Key Frontier Science Research Project, National Major Scientific Research Program, National Natural Science Foundation of China, etc.
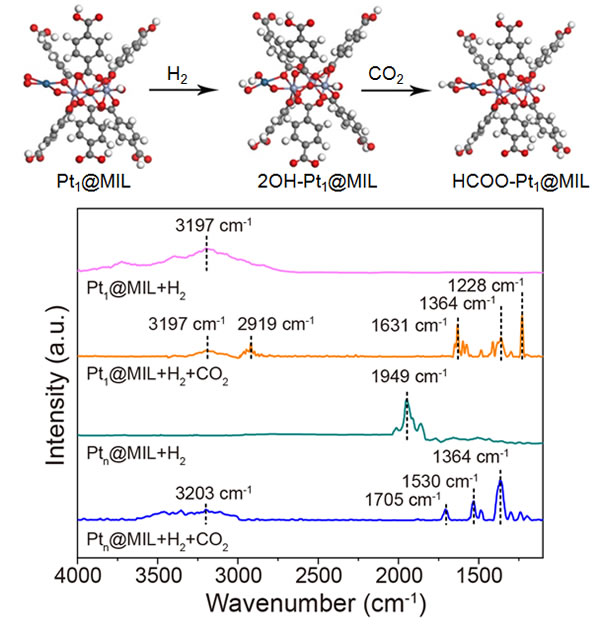
Figure: Path of Pt1 @ MIL in CO2 hydrogenation reaction
Environmental Test Machine,Impact Testing Machine,Temperature Humidity Test Chamber,Temperature Humidity Test Machine
Dongguan Best Instrument Technology Co., Ltd , https://www.dgbestinstrument.com
